PHILADELPHIA, PA, USA I January 26, 2016 I AVAX Technologies, Inc. (AVXT), a pioneer in personalized cancer vaccines, today announced the results of its Phase 1/2 OVAX study in patients with platinum resistant relapsed Stage III or IV Ovarian Cancer. The overall design is a Phase I/II, double-blind, three-dose regimen, multi-centered, trial in patients with stage III or stage IV ovarian carcinoma who have undergone de-bulking followed by intra-peritoneal chemotherapy.
Study Endpoints were treatment-emergent and related adverse events, serious adverse events, and grade 3 and 4 laboratory abnormalities for safety assessment, DTH responses to DNP-modified and unmodified autologous ovarian cancer cells. Other measured parameters were CA-125 levels and survival.
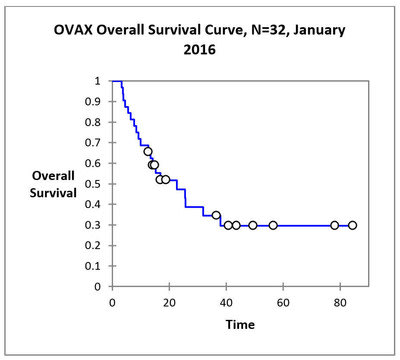
A total of 34 patients were enrolled in the study out of which 2 were lost to follow-up. 34 patients were treated at 3 doses. 12/34 are alive with 8 more than 3 years and median survival is 22.7 months. No treatment-related Serious Adverse Events have been observed.
AVAX’s management continues to be very encouraged and optimistic about the prospects of its personalized autologous cancer vaccine platform. “This study indicates that OVAX can be safely administered to patients with advanced ovarian cancer,” commented Dr. David Berd, Chief Medical Officer of AVAX Technologies. “We are also encouraged by the survival results, which appear promising in this group of very advanced ovarian cancer patients whose tumors were resistant to standard chemotherapy.”
About AVAX Technologies Inc. (AVXT)
AVAX Technologies, Inc. is a pioneer in cancer vaccine development. Although subject to the same FDA regulations as other biological products, vaccines are inherently more difficult to develop, characterize, and manufacture than most pharmaceutical products. Vaccine development and commercialization is a long complex process. Avax is a leader in terms of stage of development and commercial readiness in the immuno-oncology arena. Today, the GMP approved facility is in Philadelphia and represents one of the very few autologous vaccine approved production facilities in the world.
AVAX’s core technology platform, termed, Autologous Cell (“AC”) Vaccine Technology is a universal platform approach to individualized cancer vaccine therapy. AVAX has 2 vaccines that have completed phase 2 clinical trials, both designated with Orphan Drug Status. MVAX is a post-surgical autologous cell vaccine for melanoma, which was approved to enter a Phase III-REGISTRATION trial for the treatment of Stage 4 melanoma patients with measurable metastasis. OVAX is AVAX’s post-surgical autologous cell vaccine for Stage 3 & 4 ovarian cancer. The OVAX trial is a phase I/II trial that has completed enrollment and is in the process of gathering follow-up data on all patients. This data will be submitted to FDA when complete and will be used to file an application in support of a follow-on phase II or phase III trial. In addition, the Company’s pipeline includes a second generation autologous vaccine, developed by the inventor of MVAX and OVAX. Finally, the Company has an extensive bio-bank of lymphocytes and tumor cells from AC Vaccine-treated patients that may be of commercial interest as a key to identifying new human cancer antigens.
SOURCE: AVAX Technologies
Post Views: 92
PHILADELPHIA, PA, USA I January 26, 2016 I AVAX Technologies, Inc. (AVXT), a pioneer in personalized cancer vaccines, today announced the results of its Phase 1/2 OVAX study in patients with platinum resistant relapsed Stage III or IV Ovarian Cancer. The overall design is a Phase I/II, double-blind, three-dose regimen, multi-centered, trial in patients with stage III or stage IV ovarian carcinoma who have undergone de-bulking followed by intra-peritoneal chemotherapy.
Study Endpoints were treatment-emergent and related adverse events, serious adverse events, and grade 3 and 4 laboratory abnormalities for safety assessment, DTH responses to DNP-modified and unmodified autologous ovarian cancer cells. Other measured parameters were CA-125 levels and survival.
A total of 34 patients were enrolled in the study out of which 2 were lost to follow-up. 34 patients were treated at 3 doses. 12/34 are alive with 8 more than 3 years and median survival is 22.7 months. No treatment-related Serious Adverse Events have been observed.
AVAX’s management continues to be very encouraged and optimistic about the prospects of its personalized autologous cancer vaccine platform. “This study indicates that OVAX can be safely administered to patients with advanced ovarian cancer,” commented Dr. David Berd, Chief Medical Officer of AVAX Technologies. “We are also encouraged by the survival results, which appear promising in this group of very advanced ovarian cancer patients whose tumors were resistant to standard chemotherapy.”
About AVAX Technologies Inc. (AVXT)
AVAX Technologies, Inc. is a pioneer in cancer vaccine development. Although subject to the same FDA regulations as other biological products, vaccines are inherently more difficult to develop, characterize, and manufacture than most pharmaceutical products. Vaccine development and commercialization is a long complex process. Avax is a leader in terms of stage of development and commercial readiness in the immuno-oncology arena. Today, the GMP approved facility is in Philadelphia and represents one of the very few autologous vaccine approved production facilities in the world.
AVAX’s core technology platform, termed, Autologous Cell (“AC”) Vaccine Technology is a universal platform approach to individualized cancer vaccine therapy. AVAX has 2 vaccines that have completed phase 2 clinical trials, both designated with Orphan Drug Status. MVAX is a post-surgical autologous cell vaccine for melanoma, which was approved to enter a Phase III-REGISTRATION trial for the treatment of Stage 4 melanoma patients with measurable metastasis. OVAX is AVAX’s post-surgical autologous cell vaccine for Stage 3 & 4 ovarian cancer. The OVAX trial is a phase I/II trial that has completed enrollment and is in the process of gathering follow-up data on all patients. This data will be submitted to FDA when complete and will be used to file an application in support of a follow-on phase II or phase III trial. In addition, the Company’s pipeline includes a second generation autologous vaccine, developed by the inventor of MVAX and OVAX. Finally, the Company has an extensive bio-bank of lymphocytes and tumor cells from AC Vaccine-treated patients that may be of commercial interest as a key to identifying new human cancer antigens.
SOURCE: AVAX Technologies
Post Views: 92